Jan Van den Bossche More on Research
My research aims to explain how metabolic reprogramming regulates macrophages and disease outcome.
Macrophage Immunometabolism; where are we?
A growing number of findings highlight the crucial role of metabolic reprogramming in macrophage activation. Originally, inflammatory macrophages were described to rely on glycolysis, whereas fatty acid oxidation (FAO) and enhanced mitochondrial oxidative metabolism were linked to anti-inflammatory macrophage activation programs. We recently demonstrated that metabolic pathways are closely interconnected, exemplified by the need for glucose metabolism in anti-inflammatory as well as in inflammatory macrophages. Moreover, we observed that the role of FAO in macrophage activation is highly context-dependent. Hence, defining glycolysis as pro-inflammatory and FAO as anti-inflammatory may be an oversimplification. Using the Seahorse Bioanalyzer, we established a metabolic extracellular flux protocol that measures both glycolysis and mitochondrial oxidative metabolism in diverse macrophage subsets in real-time in one single assay. Using this technique, we demonstrated that nitric oxide-induced mitochondrial dysfunction in inflammatory macrophages prevents their r
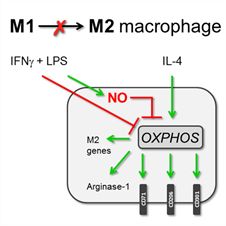
Source: Jan Van den Bossche, AMC, 2016
Macrophage Immunometabolism; where are we going?
The field of immunometabolism enters an exciting period in which key open questions will be addressed. While the metabolic characteristics and requirements of LPS+IFN?-induced and IL-4-induced macrophages are well-described in mice, the question now is how these findings can be translated to the human system? What are the metabolic characteristics and requirements of other macrophage subsets, especially in vivo? What are the underlying mechanisms that translate altered metabolism to macrophage function? Given the importance of epigenetic enzymes in macrophage activation, we previously hypothesised that epigenetics could serve as a bridge between metabolism and function.
By unravelling these distinct questions our research will help to answer whether targeting of macrophage metabolism could be used for future therapy.
Supported by a senior postdoctoral fellowship, awarded by Netherlands Heart Foundation, we will target particular immunometabolic circuits in atherosclerotic plaque macrophages to improve their function and disease outcome. In a parallel project, we will delineate the metabolic characteristics and requirements of tumor-associated macrophages in distinct cancer patients and mouse tumor models.