Sue Gibbs
About
Sue Gibbs is professor in Skin and Mucosa Regenerative Medicine. She is PI at the Department of Molecular Cell Biology & Immunology and also in the Department of Oral Cell Biology, ACTA. Her entire career has focused on animal alternative methods, in particular in developing human healthy and disease models drug discovery, pre-clinical studies and safety testing. She combines cutting-edge research in cell biology and immunology with advances in tissue engineering. Current focus lies with developing next generation immune competent tissue engineered constructs to investigate the (patho)physiology of adverse scar formation and to investigate similarities and differences between tolerance, allergic and irritant contact dermatitis with the aim of identifying novel drug targets for personalized as well as general therapeutic strategies. Recently, her research has extended into the field of hair follicles and importantly ‘’organ-on-a-chip’’, in particular immune competent skin-, gingiva-, gut,- lymph node- and melanoma- “on-chip”. Sue Gibbs was awarded numerous grants from EU, NWO, TTW, Dutch Burns foundation, in collaboration with partners from industry.
Research Line
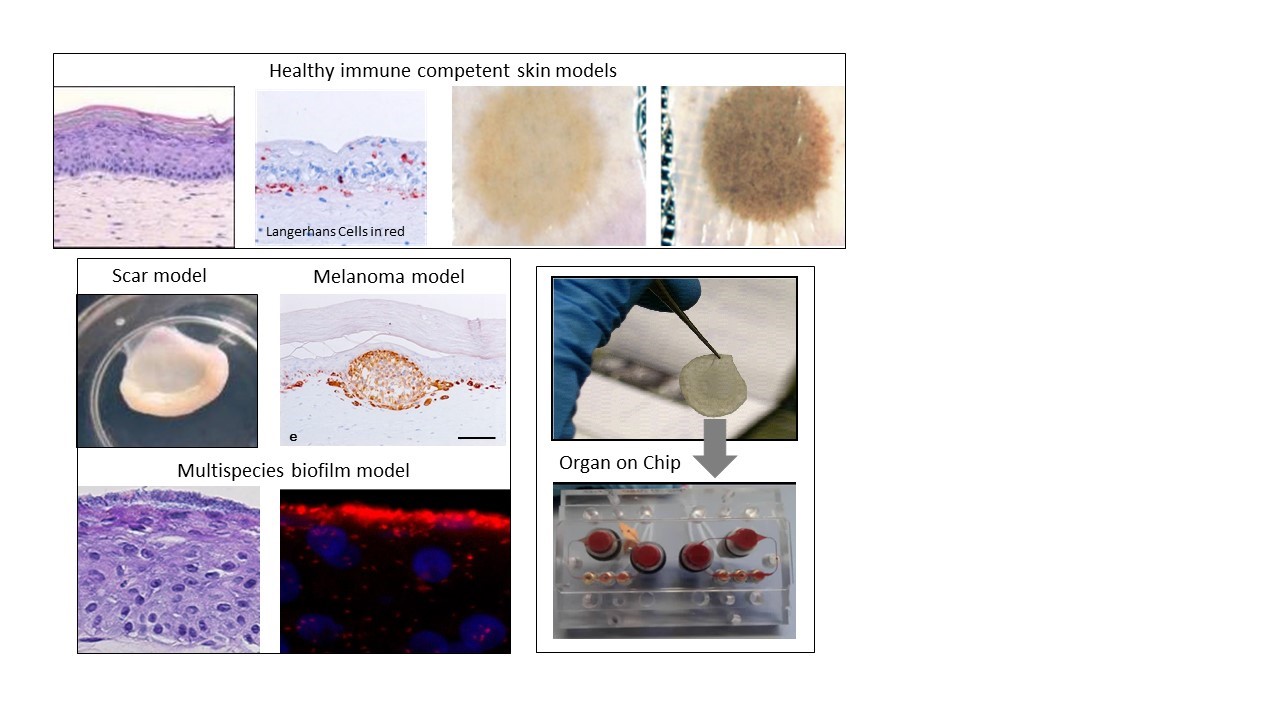
Human healthy and disease model technologies: Immune competent Skin-on-Chip for risk assessment and cancer research: NextSkin project Our aim is to develop the next generation immune competent human skin models which closely represent healthy and diseased skin, thus providing a platform for safety and efficacy testing of drugs and consumer products which come into contact with our skin. Our focus is on fibrosis, cancer, allergy and tolerance. Cell types which can currently be built into our organotypic models include epithelial cells (keratinocytes, melanocytes, Langerhans cells, melanoma), dermal cells (fibroblasts, myofibroblasts, hair follicles, blood and lymph vessel endothelial cells, dendritic cells, monocytes, macrophages) and adipose tissue cells (mesenchymal stromal cells, adipocytes and endothelial cells). These models are being incorporated into micro-physiological bioreactors, ‘’organ-on-a-chip’’, to create immune competent skin-, gingiva-, gut,- lymph node- and melanoma- “on-chip”.
Inflammatory skin and oral mucosa disease
Research is aimed at identifying the mechanisms involved in skin and oral soft tissue homeostasis and inflammation. Examples of our research questions include: why does a deep skin wound heal with a bad scar formation whilst oral wounds heal with relatively little scar? Is this to do with intrinsic properties of the cells or is this due to extrinsic factors e.g.: saliva, microbiome? With the aid of normal skin / gingival equivalents, and in vitro hypertrophic scar and keloid scar equivalents the mechanisms of scar formation can be investigated. Another major research question is: why does first contact with an allergen (e.g. Nickel) via the skin often result in sensitization whereas first contact via the mouth with the same allergen results in tolerance? With the aid of skin and gingival equivalents containing integrated Langerhans Cells, innate and adaptive mechanisms involved in sensitization versus tolerance and pathogen infection are being investigated.
Key publications
1. Shang, L., et al., Commensal and Pathogenic Biofilms Alter Toll-Like Receptor Signaling in Reconstructed Human Gingiva. Front Cell Infect Microbiol. 2019 Aug 7;9:282. doi: 10.3389/fcimb.2019.00282. eCollection 2019
2. Vahav, I., et al., Reconstructed human skin shows epidermal invagination towards integrated neopapillae indicating early hair follicle formation in vitro. J Tissue Eng Regen Med. doi:10.1002/term.3039 2020
3. Limandjaja, G.C., et al., Characterization of In Vitro Reconstructed Human Normotrophic, Hypertrophic, and Keloid Scar Models. Tissue Eng Part C Methods, 2018. 24(4): p. 242-253.
4. Monsuur, H.N., et al., Endothelial cells enhance adipose mesenchymal stromal cell-mediated matrix contraction via ALK receptors and reduced follistatin: Potential role of endothelial cells in skin fibrosis. J Cell Physiol. 2018 Oct;233(10):6714-6722. doi: 10.1002/jcp.26494. Epub 2018
5. Kosten, I.J., et al., MUTZ-3 derived Langerhans cells in human skin equivalents show differential migration and phenotypic plasticity after allergen or irritant exposure. Appl. Pharmacol, 2015. 287(1): p. 35-42.