Jack van Horssen Group leader
Throughout his scientific career Jack van Horssen has been interested in understanding the pathogenetic basis of neurodegenerative disorders. He obtained his PhD at the Radboud University Nijmegen in 2005 on the expression and role of heparan sulphate proteoglycans in Alzheimer's disease in the group of Prof. dr. D. Ruiter, Dr. R de Waal and Dr. M. Verbeek. Thereafter, he started as a postdoctoral scientist in the group of Prof. dr. H.E. de Vries working on extracellular matrix alterations in MS brain tissue. In 2010 he received the MS Fellowship that allowed him to continue his scientific work on the identification of molecular pathways underlying impaired mitochondrial metabolism and reactive oxygen species production in MS and to examine the potential of antioxidant and mitochondrial protection to counteract oxidative stress and improve mitochondrial function. The approach the group uses is to assemble information obtained from careful neuropathological examination of MS brain tissue and translate these findings into experimental in vitro and in vivo models and vice versa. Together with many collaborators the group has published over 80 papers in peer-reviewed journals. Their work is funded by collaborative grants from pharmaceutical companies, Amsterdam Neuroscience, Progressive MS Alliance, Dutch MS Research Foundation and the National MS Society. Jack van Horssen is currently appointed as an associate professor at the Department of Molecular Cell Biology and Immunology and a visiting professor at the University of Hasselt, Belgium. He has a strong teaching profile and is intimately involved in the bachelor program of the study of Medicine.
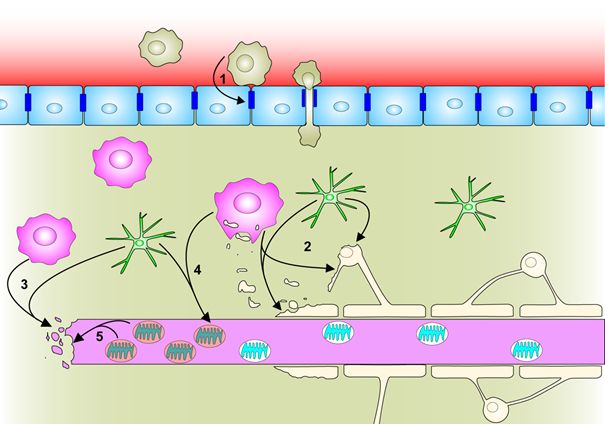
Figure 1. Reactive oxygen species contribute to various processes underlying multiple sclerosis pathogenesis (van Horssen et al. Radical changes in neuroinflammation. Biochim. Biophys. Acta. 2011)
Upon adhesion to activated brain endothelial cells, monocytes produce ROS, which induce tight junction gap formation and cytoskeleton changes thereby facilitating transendothelial monocyte migration (1). Macrophage and activated microglia-derived ROS induce demyelination and oligodendrocyte cell death, facilitate myelin degradation (2) and mediate axonal degeneration (3). Inflammatory cytokines and ROS contribute to mitochondrial dysfunction and accumulation of impaired mitochondria in chronically demyelinated axons, due to increased intra-axonal energy demand (4). In these chronically demyelinated axons, mitochondrial ROS production further contributes to axonal injury (5).
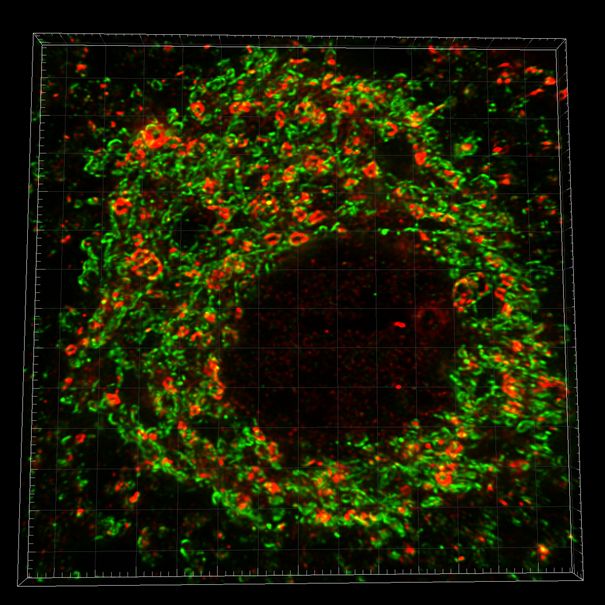
Figure 2. Ultra-resolution image of the neuronal mitochondrial network in a paraffin section