Microscopy Unit MCBI
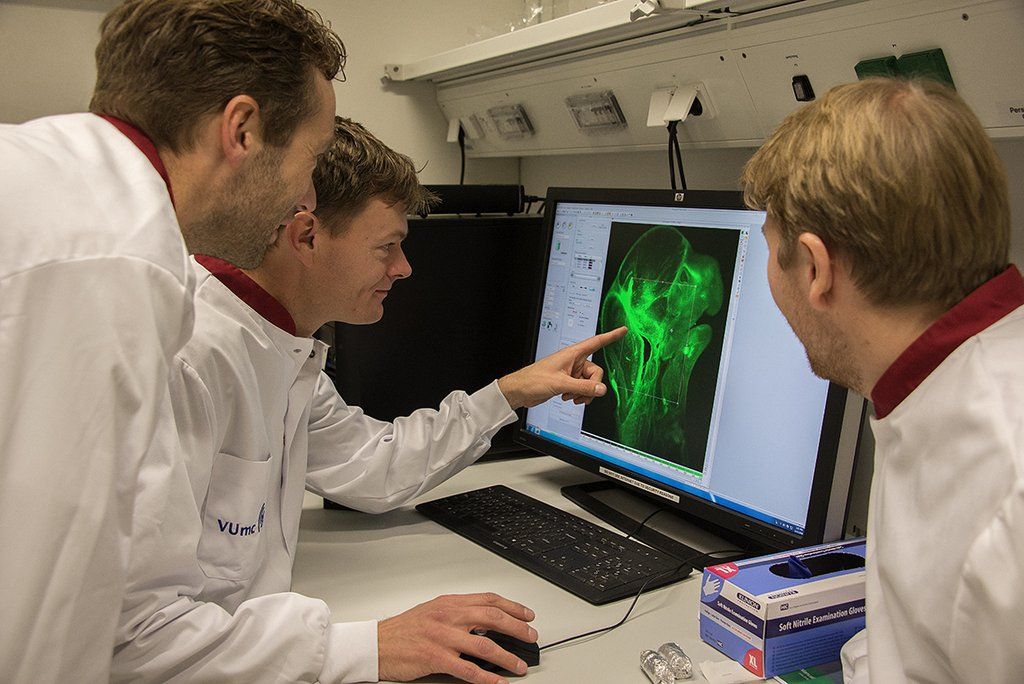
Training and support is provided by two employees. Specialized usergroup meetings for microscope techniques and analysis software are organized for sharing expertise between researchers from different institutes and departments.
Together with departments Physiology, ANW and MOVE, the MCBI is embedded in the AOI2M microscope facility with the advanced microscopy techniques. For information about the facility: www.AO2M.amsterdam.
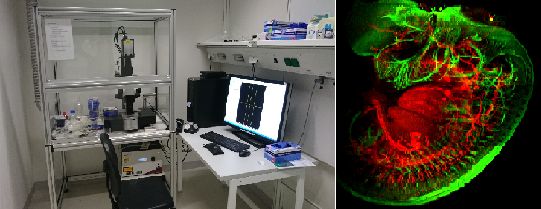
Ultra microscope II from Lavision Biotec (superK Extreme white light laser NKT Photonics; Andor Neo sCMOS camera) / Embryo, beta-3-tubulin, J. Koning

Trimscope LaVision Bio Tec ( Ti Saph laser tunable 700-1000nm; 3,4W, femtosec pulsed) / In vivo lymphnode: blue SHG signal of collagen, green autofluorescence (L. v.d. Steen/ E. Hendrikx)
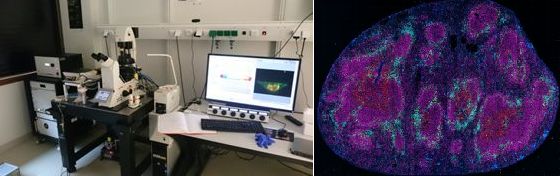
Leica TCS SP8 gated STED 3X

CM100 Philips with Morada G2 camera / Skin tissue, N. Paauw
5. Life cell imaging with automatic drift compensation (ZDC)and simultaneous imaging on several locations.
Fluorescence combined with DIC. Suitable for time lapse experiments with cells in culture. OlympusIX81 with climatisation chamberl (37°C,CO2 and humidity controlled) and motorized table.
6. Bright field and fluorescence microscopes for stained slides: complete overviews with automatically high resolution imaging is possible due to the wide range of objectives (1,25-100x) combined with motorized stage and suitable software.
Leica DM6000 with DFC 320 and DFC350 FX camera; Zeiss Imager D2 with AxioCam HRM camera; Zeiss Scope A1
7. Multihead bright field microscope for teaching or discussion possibilities with several people.
Bright field Nikon Y-THM
8. Several Bright field (phase contrast) and fluorescence microscopes in culture rooms ML1 and ML2.
Suitable for well plates (inverted micropes) with imaging possibilities or manual cell counting.
Upright fluorescence- and bright field microscope with large objective range and high resolution color camera Nikon E800
The MCBI Microscopy Team
Prof. dr. Elga (H.E.) de Vries
Professor at Department Molecular Cell Biology & Immunology
Advisor of microscopy unit, board AOI2M facility
Nanne Paauw
Operator. Speciality: EM, superresolution microscopy and light sheet microscopy